We have a new paper in The Journal of Physical Chemistry C, written by Dr. Bebi Patil. It deals with composite solid electrolytes (CSEs) based on (1) a Li-conducting filler (LATP) in (2) a polymer matrix (PEO + LiTFSI). This work demonstrates that engineering fillers to have space-filling shapes has a profound positive impact on conductivity of the resulting CSE. Our approach was to engineer a micron-scale filler with a hollow-sphere morphology (shown below) because hollow spheres fill space efficiently and shift agglomeration to the level of the secondary particles. Also the hollow spheres are porous and have both an inner and outer surface.
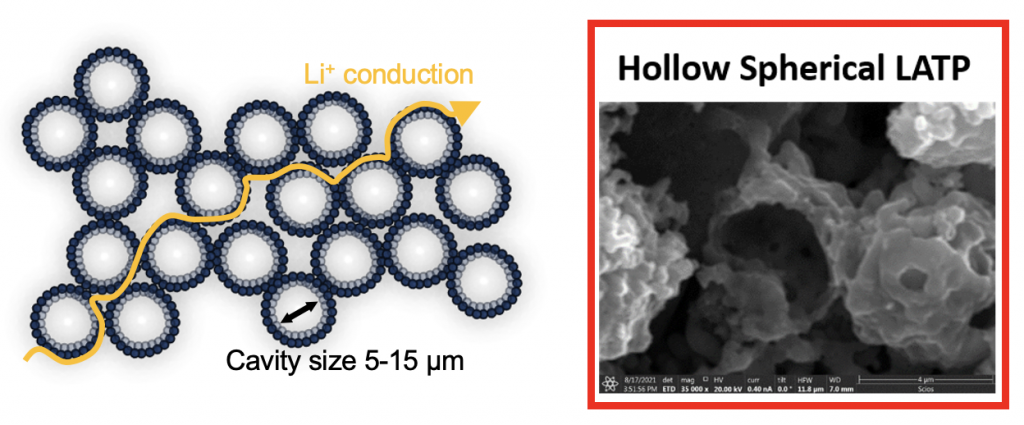
With CSEs, it is of paramount importance for the ceramic filler to present maximum surface area to the polymer matrix. This is typically done by exploiting the high surface area to volume ratio of nanomaterials. To achieve a percolated Li-conducting interphase across the electrolyte, many groups have engineered fillers in a nanofiber or nanorod morphology. However, our micron-scale hollow sphere material performed as well as or better than nanoscale materials. It had a near-record room temperature conductivity (1.64 × 10-4 S/cm) for a PEO/LATP-based CSE. (This is a good start, but conductivity still needs to be higher for CSEs to be widely used in battery products.)
Li metal batteries based on CSEs are desired because polymer processing would be simpler to integrate into battery manufacturing than the high temperature methods required for ceramic solid electrolytes. Also, polymers make good interfaces with battery active materials. The challenge is that their Li conductivities are generally much lower than other solid electrolytes (like sulfides or garnets). Including a filler material improves the conductivity. There are several reasons for this, some of which are not completely understood.

Northeastern student Marisol Chang Zapata designed us a wonderful cover image!
