We have a new paper out, dealing with our long-term effort to develop extremely inexpensive and safe batteries for large-scale use. The full title is “Rechargeability and economic aspects of alkaline zinc-manganese dioxide cells for electrical storage and load leveling.” The idea driving the research is that since DOE cost targets for grid-scale storage are very low ($100/kWh), battery design should begin with the cheapest and safest materials possible, instead of trying to adapt high-power lithium-ion battery designs.
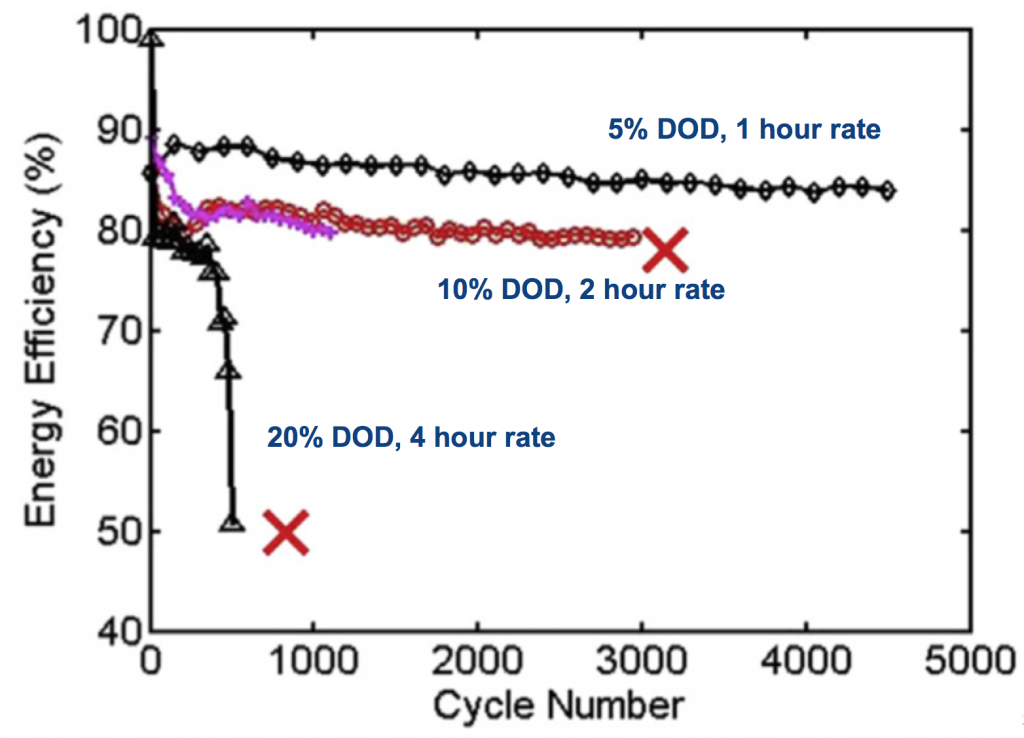
Zinc and manganese dioxide fit that bill, and what the paper shows is that by reducing the depth of discharge (DOD) to some fraction of the full capacity, long cycle life can be achieved at extremely low cost. The plot below shows that at 10% DOD over 3000 cycles are typical, while at 20% DOD it is over 500. These are base designs, focusing on electrode construction, materials, porosity, etc, with no additives or specialty materials. Assuming one cycle a day, this is an 8.5 year lifespan at 10% DOD.
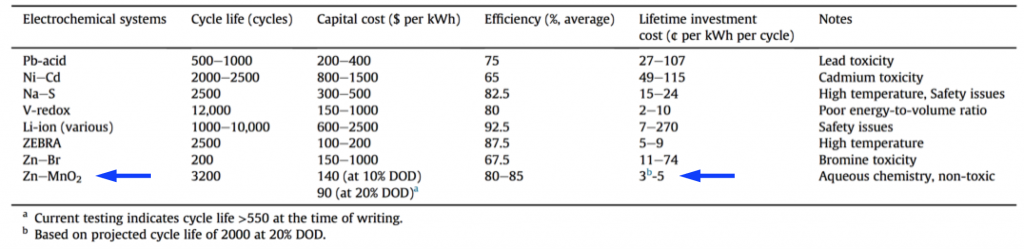
Cycling a fraction of the DOD like this allows a long lifespan, while keeping the cost low. This shallow-cycled Zn-MnO2 battery comes in very low in lifetime investment cost, 2-5 ¢/kWh/cycle. Using numbers taken from the literature, this is in or below the projected range of vanadium-redox and sodium-metal-halide (ZEBRA) batteries, while being low-temperature and generally lower complexity than these. An extra benefit is that since only a fraction of the Zn-MnO2 capacity is cycled, the reserve capacity can be used in emergencies.
Over the last few years we’ve developed these types of batteries in many shapes and sizes, supported by DOE’s ARPA-E program as well as other entities like the utility Con Edison. A couple of battery racks in the CUNY lab are shown below. A little over a year ago Urban Electric Power LLC was spun out, and in their battery labs a few blocks away on 127th Street they’ve been developing the batteries for commercialization, and refining the design for higher power and longer life.
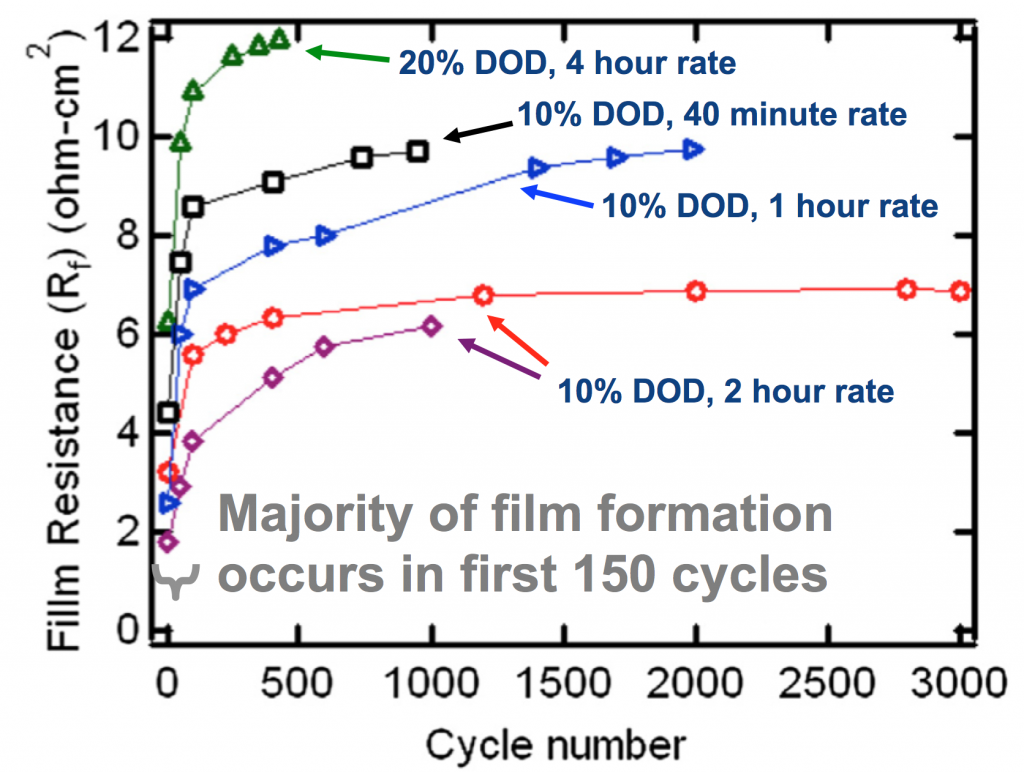
Our analysis in the paper addresses evolution of the batteries during their lifetime. If you notice above, at 10% DOD the efficiency falls from near 90% to near 80% during cycling, due to a kind of “formation” of the materials. The analysis, which was Nilesh Ingale’s doctoral thesis, is too dense to describe in full but a combined modeling+experimental effort shows that the MnO2 active particles in the cathode become coated with hetaerolite (ZnMn2O4) and this resistive film drops performance. Below you see this resistivity plotted against cycle number, showing the effect mostly occurs in the first 150 cycles. Preventing this will help efficiency, but could also impact how the batteries are made and lower cost.