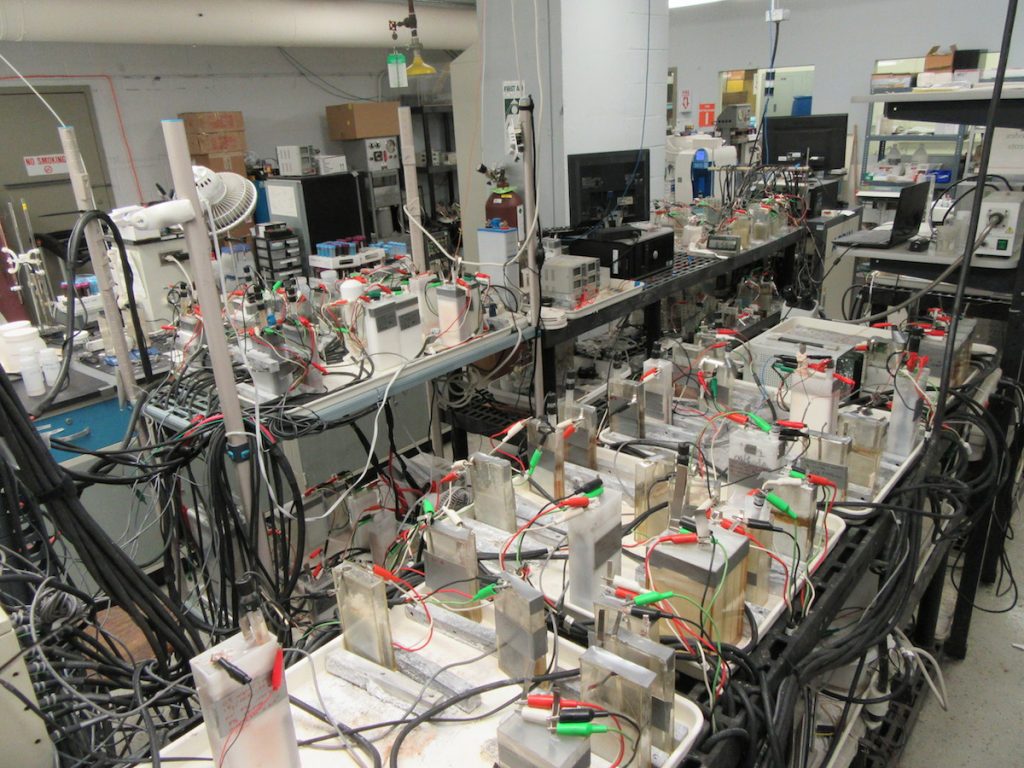
… and fellow Scott Barton student, Dr. Nicholas Hudak. At lunch did we talk about batteries and electrochemistry, boring our significant others? Only a little!
Category Archives: Uncategorized
Happy New Year
How many peer reviews do you do in a year?
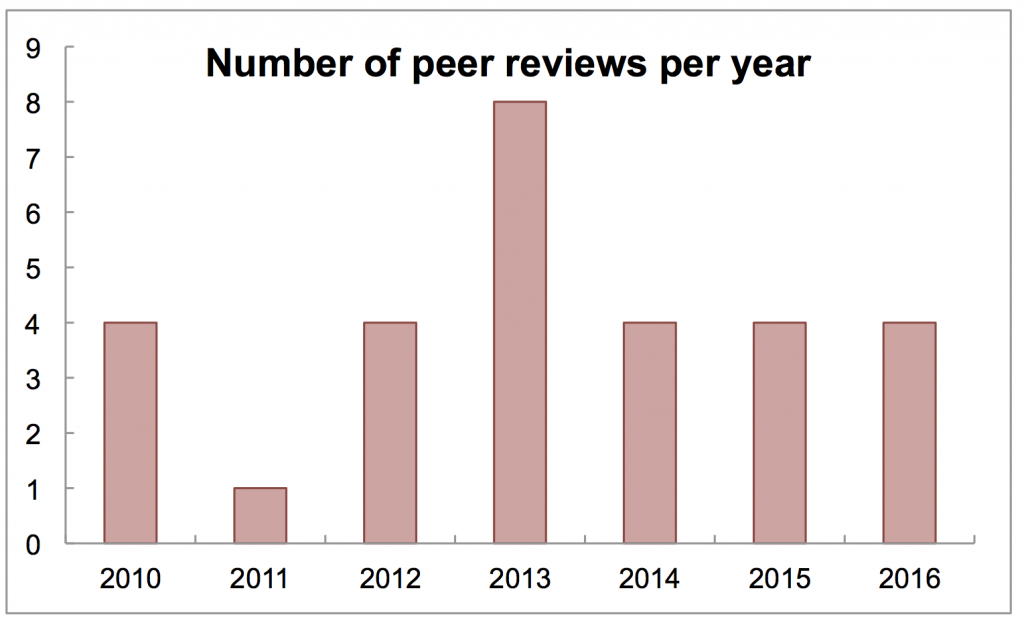
I have a folder where I put all the files I create while peer reviewing a manuscript. This weekend I finished the fourth of 2016, and I usually assume the fourth will be the last. I wondered if that was always true. By counting up the files in this folder, I was able to see how many I had done each year, plotted below.
Four is in fact the most common number (the “mode” in this dataset, if you will). But what happened in 2013 and why did I do eight? By the way, I also see one of those had three revisions.
Update October 2016: I spoke too soon. I’m going to hit nine this year. You’re welcome, editors.
Update January 2017: Final tally: eleven. I believe in my heart of hearts the late-breaking rush was due to my tempting fate with this very post.
The beauty of dye-sensitized solar cells
Check out these photos of EPFL’s SwissTech Convention Center, which has a facade covered in dye-sensitized solar cells (DSSCs). The archetypal dye for a DSSC is a ruthenium complex called “black dye.” Dyes like actually have an enchanting purple-brown-black color, which isn’t totally black. But you can theoretically use any molecule that will inject an electron into a semiconductor when hit by light, and I just did a bit of hunting around and found a company called Dyenamo that specializes in different colors. The bottom picture is from C&EN, which has an article on non-silicon solar cells this week.
New PNNL paper on zinc/manganese oxide energy storage
I’ve had quite a few people email asking what I think about the new paper Reversible aqueous zinc/manganese oxide energy storage from conversion reactions in Nature Energy, by authors at PNNL. Understandable, since I spend a lot of my time talking about Zn and MnO2 for electrical storage. Fair!
First: unfortunately the work is being marketed (by PNNL) with a terrible graphic of a Platonic ideal supergreen™ battery that sits in a sunlit field and emits rays of light that save the world, but that’s pretty standard for battery research these days. Once the PR department gets ahold of it, you’re waist-deep in pictures of suns, windmills, iPhones, and Teslas. Most people, even most scientists, don’t understand the many levels of hierarchy involved in battery design and engineering, so I try to overlook these kinds of silly Photoshop excursions.
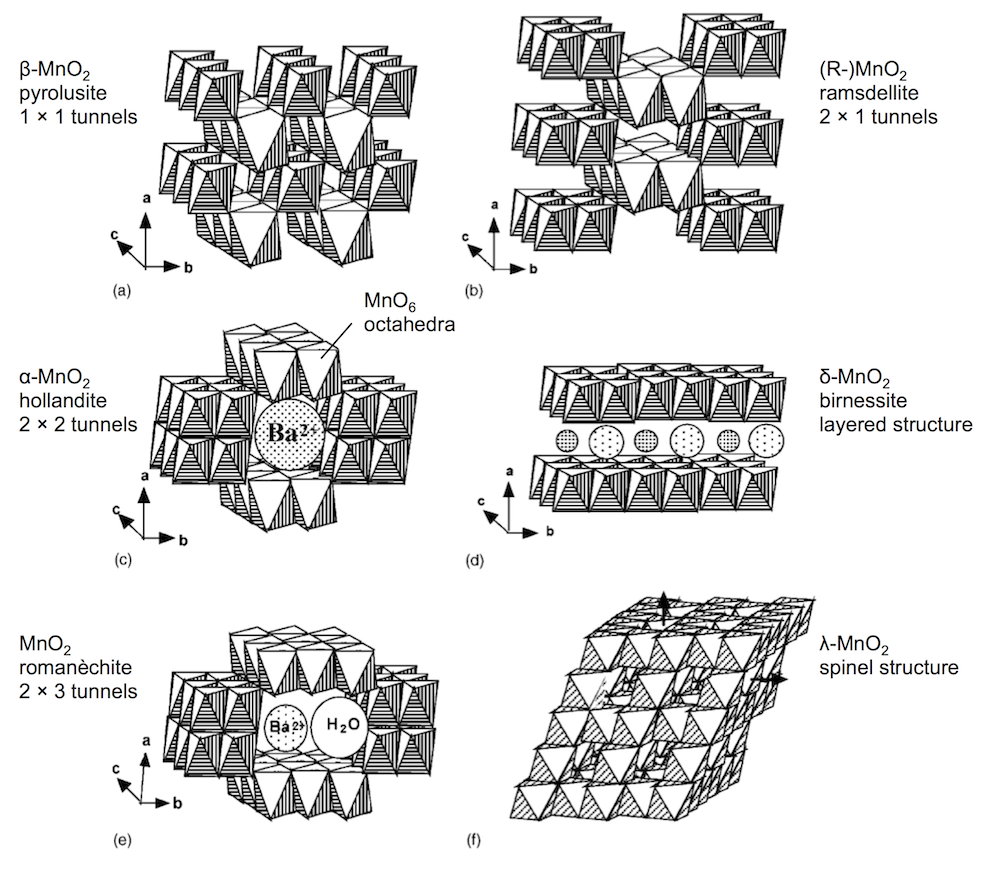
Second: the innovation of the paper is that they are making a rechargeable Zn-MnO2 battery in a mildly acidic electrolyte, and getting good cycle life. The way they’re doing this is by using α-MnO2 as their cathode active material. MnO2 comes in several polymorphic forms, some of which you can see below. (I adapted this figure from a paper by Poinsignon.) They are built from MnO6 octahedra, but can distinguished by the tunnel structures in the crystal.
A lot of my recent work has focused on the polymorph γ-MnO2, which is an intergrowth of (a) and (b) above. The PNNL work makes an interesting discovery about α-MnO2: they see the α-MnO2 going through a conversion reaction to MnOOH, which is somewhat unexpected. As you can see in the figure above, α-MnO2 is usually thought of as a host structure, to intercalate guest ions (like Ba2+). They then see that the surface of the MnOOH is coated with a large flake-like material that originates with the sulfate electrolyte, ZnSO4[Zn(OH)2]3 · xH2O. In this respect, the reaction is a bit reminiscent of a lead-acid battery, which also involves a sulfate film.
The paper is very interesting in that it provides unexpected evidence of α-MnO2 acting in the manner of a conversion reaction. (And that’s why that term is important and shows up in the paper’s title.) Also the zinc hydroxyl sulfate flake-film is a tantalizing look at what could be a very complex cathode reaction. And I’m a sucker for complex electrochemical reactions, as I hope you know. The test bed for the research was a CR2032 form factor, which is the kind of battery that goes in my running watch. So, the picture the PR machine and the science press are painting (with that world-saving battery up above) is a bit overblown, but the electrochemistry research underpinning this paper is extremely interesting, and I hope to see more.