Some exciting news: we have a new paper out in Nature Communications featuring a deep cycling manganese dioxide (MnO2) cathode. This cathode is aqueous, operating for thousands of cycles in KOH electrolyte, at essentially the full 2-electron capacity of MnO2, which is 617 mAh/g-MnO2. MnO2 is abundant, inexpensive, and non-toxic, making it an ideal basis material for large-scale batteries at the scale of the power grid. Batteries on this scale will make widespread use of solar and wind power possible in the future. Because the electrolyte is water-based, the resulting battery is inherently safer than Li-ion, which relies on a highly flammable electrolyte. Fires caused by Li-ion batteries in small devices like cell phones are a generally accepted risk, but for massive stationary batteries located in power grid infrastructure the risk assessment recommends using a safer, water-based battery chemistry.
Some exciting news: we have a new paper out in Nature Communications featuring a deep cycling manganese dioxide (MnO2) cathode. This cathode is aqueous, operating for thousands of cycles in KOH electrolyte, at essentially the full 2-electron capacity of MnO2, which is 617 mAh/g-MnO2. MnO2 is abundant, inexpensive, and non-toxic, making it an ideal basis material for large-scale batteries at the scale of the power grid. Batteries on this scale will make widespread use of solar and wind power possible in the future. Because the electrolyte is water-based, the resulting battery is inherently safer than Li-ion, which relies on a highly flammable electrolyte. Fires caused by Li-ion batteries in small devices like cell phones are a generally accepted risk, but for massive stationary batteries located in power grid infrastructure the risk assessment recommends using a safer, water-based battery chemistry.
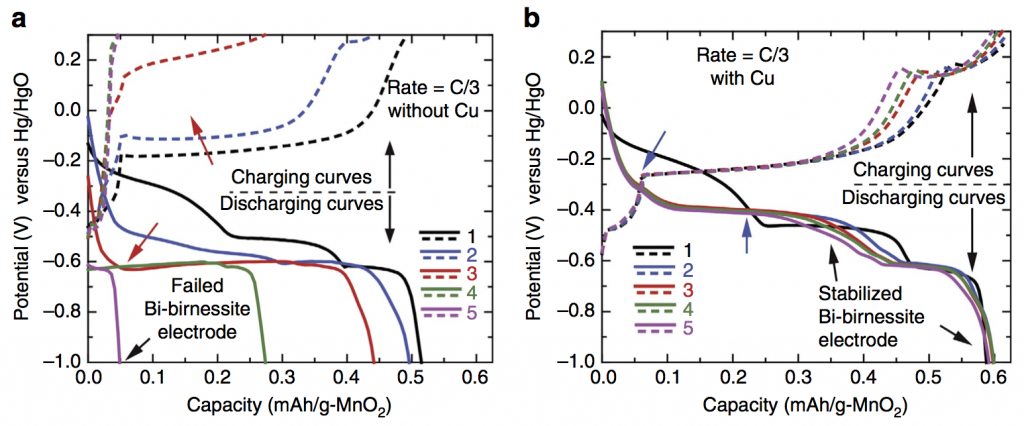
The paper title is Regenerable Cu-intercalated MnO2 layered cathode for highly cyclable energy dense batteries, and the innovation that makes everything possible is intercalating copper (Cu) into bismuth-modified δ-MnO2 (birnessite). Bismuth-birnessite was discovered by Ford Motor Company in the 1980s, but it was never known how to use it at high areal capacity. The first five cycles of a cathode with no copper (a) and with copper (b) are shown below. Without copper the cathode failed by the third cycle.
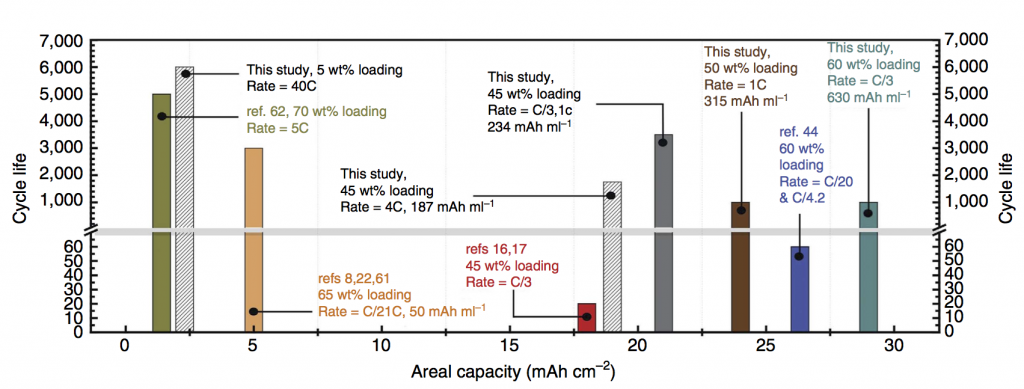
Gautam Yadav discovered this copper intercalation here at the CUNY Energy Institute during the final year of our ARPA-E project. The reason it is a big discovery is this: Bi-birnessite will cycle nicely under some conditions, for example at low mass loading or with a very thin electrode, i.e. situations with low areal capacity. In contrast, we achieved both high cycle life and very high areal capacity. Areal capacity is measured in mAh/cm2, and achieving high areal capacity is critical for packing a lot of electrodes together with high energy density. Any practical battery requires high areal capacity (as opposed to batteries meant as academic exercises or low-level proofs of concept).
The plot above puts areal capacity in context. One recent report (ref 62) achieved 5,000 cycles, but at a small areal capacity of 1 mAh/cm2. Another report (ref 44) successfully achieved 26 mAh/cm2, but with only 60 recharging cycles. By using Cu-intercalated MnO2 we achieved 6,000 cycles at 2.5 mAh/cm2 and 1,000 cycles at 28 mAh/cm2. Battery modeling suggests this opens up a design pathway to 200 Wh/L, equal to the energy density of some kinds of Li-ion battery, for an aqueous Zn-MnO2 battery.
Press release from CCNY. Press release from CUNY.