Electrochemistry is fundamentally an interfacial science, dealing with reactions that happen at the interface between an electron conductor and an ion conductor. In many cases, including most practical batteries, this is a solid-liquid interface. Most batteries are consequently designed to be composed largely of interface. Often compressed particles of active material make up the electrodes and liquid electrolyte fills the pore space between the particles. The goal of battery engineering revolves around questions of either energy or power, and what value of these can be achieved in a device of a given mass, volume, or cost. Thus some metrics of battery success dealing with energy are: specific energy (Wh/kg), energy density (Wh/L), or capital cost ($/kWh). High specific energy is crucial for automotive batteries, while low capital cost is most important for large, stationary batteries at the building or grid scale.
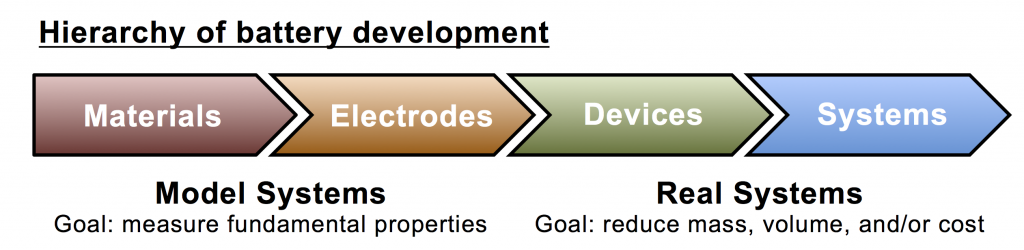
Another fundamental feature of electrochemistry is that it by necessity involves the interplay of two electrodes, an anode and a cathode. Experimental techniques exist to test electrodes in isolation, but in practical devices such as batteries the electrodes usually interact. Device performance is determined not only by the performance potential of each electrode, but also by the effect of each electrode on the other. Because of this and the need to maximize interface, battery design is hierarchical (see fig. above): (i) active materials are chosen due to their voltages and rate capabilities; (ii) the active materials are formed into useful electrodes, which may have high surface area; (iii) the two electrodes are integrated into a device; and (iv) devices are connected (in series or parallel) to form a system, if needed.
This hierarchy requires specialized characterization at every level. There has long been an established toolkit for electrochemical materials characterization, but true device-level characterization is only now coming into its own as techniques are developed to collect microscopic data from within sealed batteries. Device-level knowledge beyond voltage/current (E/I) data is extremely powerful and promises to advance understanding into the complex mechanisms that occur inside cycling batteries. An overreliance on materials-level characterization ignores conditions arising from device-level aspects. One example is electrolyte concentration at the outer radius of a AA battery, which falls from its initial value during discharge, and is thus different than the same concentration in a D battery, which rises due to a much longer transport path to the anode. With materials-level characterization there is also the danger for a fallacy of composition, in which remarkable capabilities of the materials are inferred to the device without justification, based on a misunderstanding of battery design principles.